WELCOME TO
Psychedelic Alpha
Independent data-driven reporting, analysis and commentary on the psychedelics space: from business and drug development through to policy reform and culture.
Join thousands of psychedelic insiders…





Loved by 10,000+


Comprehensive Psychedelic Data
Explore Our Curated Psychedelic Sector Data Bank.
Access curated data on psychedelic patents, drug development, and policy reforms.
- Patent Trackers
- Drug Development
- Policy Reforms
- Financing Trends
- Stock Lockups
- Sector Insights
Cited By Over 500+ Leading Brands
and Publications Worldwide.








Expert Advisory and Incubation for Psychedelic Ventures.
Providing consultancy, advisory, and incubation services for investors and entrepreneurs in the psychedelic sector.
Successful Projects
0
+
Years Industry Experience
0
+
Client Satisfaction
0
+
Stay Informed, Inspired, and Ahead with Premium Content and Resources.
Unlock Exclusive
Psychedelic Insights with Pα+

Exclusive Insights
Gain access to in-depth articles, interviews, and analyses on psychedelic developments.

Regular Bulletins
Stay updated with timely bulletins covering major stories and breakthroughs.

Insider Interviews
Hear directly from industry leaders and experts shaping the psychedelic landscape.

Quick-Take Analyses
Receive swift analyses of significant trial results, financing rounds, and policy reforms.

Comprehensive Library
Explore our extensive back catalogue and Library of resources.
Our latest insights
In this Issue
- Psychedelics Insiders Draw Up Trump 2.0 Wishlist
- Rick Doblin Reflects on Lykos’ Strategy as MAPS Settles with FDA
- Polish Medical Research Agency Set to Provide PLN 16M ($4M) to Psilocybin Study
- VA Issues Note on Psychedelics for PTSD; Officially Funds Psychedelics Study
- Alleged Killer of UnitedHealth CEO Apparently Interested in Psychedelics
- Other Stories including: MDMA Plus Massed Exposure Therapy Protocol Published; Pollan Pushes Back on RFK Jr. Hype; Greenbrook TMS Stock Slumps Following Q3 Earnings; 30 Magic Mushroom Hops Shutter in Ontario; Expression of Concern Issued Over MAPS/Lykos Journal Article; Relmada Therapeutics’ Phase 3 MDD Drug Fails; and more…
***
Psychedelics Insiders Draw Up Trump 2.0 Wishlist
As some of us draw up our wishlists for Santa’s perusal (though, apparently Saint Nicholas has been found a little worse for wear in Turkey), some psychedelics insiders are noting down their hopes for the incoming Trump administration.
Since the U.S. elections last month, I have been speaking with people from all corners of the psychedelics field to better appreciate what they’re hoping the new government might actually do when it comes to psychedelics-related issues.
Here’s a shortlist…
Right to Try and Expanded Access
- A common MVP (minimum viable policy, if you will) mentioned by insiders is enhancing access to psilocybin and MDMA via the federal Right to Try Act.
- That Act allows patients diagnosed with a life-threatening condition who have exhausted existing treatment options to access certain investigational drugs.
- However, the Drug Enforcement Administration (DEA) has prevented patients from accessing Schedule I drugs like psilocybin and MDMA through this process (see, for example, AIMS v. DEA).
- Some advocates and lawmakers have sought to rectify this situation by introducing a clarification act, which would specify that access to schedule I substances should also be considered under the pathway.
- Insiders hope that efforts to shore up the accessibility of psychedelics through this route might firm up during the next administration.
- Expanded access programs were discussed in a similar vein, with some describing them as approximating a real-world trial.
A ‘Cole Memo’ for State-Regulated Psychedelics
- Another common wishlist item is some sort of agreement from the federal government to not interfere with state-regulated psychedelics programs.
- In some ways, that would be similar to the Cole Memorandum issued in 2013 by Deputy Attorney General James Cole under the Obama administration. That memo said that, given its limited resources, the Department of Justice did not intend to enforce its federal prohibition of marijuana in those states that had adopted more liberal policies, except in certain circumstances. That was a boon for state-regulated programs, which could operate without the federal government and its agencies breathing down its neck quite so threateningly.
- But, psychedelics advocates are hoping for something a little firmer than a memo, which didn’t really have any true legal clout.
- Getting something into law, then, would be better. In late 2023, California Democrat Robert Garcia introduced the VISIONS Act, which would have prohibited the use of federal funds to prosecute state-legal psilocybin use (see our September 2023 coverage for more). Advocates are hoping a new administration might put something similar into law.
- It’s unclear how amenable the incoming administration will be to this. Of course, Republicans have focused on ‘states’ rights’, especially in recent policy decisions around thorny issues like abortion laws. But it is worth remembering that Jeff Sessions, Trump’s Attorney General during his first term, abruptly nixed the Cole Memo.
Concessions at the FDA
- Insiders also hope to see psychedelics-related concessions at the FDA, with a whole wishlist of its own in that regard.
- Some hope the agency will simply reverse its rejection of Lykos Therapeutics’ MDMA-assisted therapy New Drug Application (NDA), though that was not expressed as a realistic expectation by many.
- Outside of revisiting specific decisions, some insiders told me they hope the FDA will change how it weights different elements of trial design and results when making decisions on whether to approve drugs.
- Several people told me that they hope to see the agency focus less on functional unblinding and durability, for example.
- Others hope to see the agency shift more of its data requirements and review to the post-marketing environment, allowing drugs to come to market on the basis of a characterised safety profile and perhaps only a signal of efficacy.
- (We have discussed these topics previously, such as in Psychedelic Drug Development Under Trump 2.0: Health Nominees Signal Uncertain Future and Tripping Over Trump: Will the New Administration Embrace Psychedelic Exceptionalism or ‘Just Say No’?)
NIH Funding for Psychedelics
- As we covered recently, some researchers in the field are concerned about the future of NIH funding for psychedelics projects, which has become an increasingly important source of cash for both preclinical and clinical work…
Sign-in or join Pα+ to continue reading this Issue of the Psychedelic Bulletin…
Join Pα+ Today
Independent data-driven reporting, analysis and commentary on the psychedelics space: from business and drug development through to policy reform and culture.
Already a member? Log In
✓ Regular Bulletins covering key topics and trends in the psychedelics space
✓ Regular articles and deep dives across psychedelic research, policy and business
✓ Interviews with insiders
✓ Monthly interactive database and commentary on psychedelic patents
✓ Quick-take analysis of major developments
✓ A Library of primers and explainers
✓ Access to our full back catalogue
In our latest interview, Psychedelic Alpha’s Josh Hardman speaks with Javier Muniz, MD, who recently left his post as Associate Director at the FDA’s Division of Psychiatry. In that role, Muniz was at the heart of the agency’s psychedelics learning journey and helped formulate its view on thorny topics in psychedelic drug development like functional unblinding and the role of psychotherapy.
Muniz has been on our radar for quite some time as a leader within the agency on the topic of psychedelics, so we were pleased to speak with him now that he is able to discuss these matters more candidly. Since leaving the agency, Muniz has begun offering consultancy services via his firm PsyNova Solutions, and shortly after we conducted this interview he was appointed as VP of R&D at psychedelic drug developer MindMed. He very much intends, then, to continue engaging with the psychedelics field.
The interview itself is far-reaching. We begin by discussing the FDA’s psychedelics-related trip of discovery, which includes Michael Pollan’s best-selling book but also standing-room-only presentations at conferences and internal symposia that saw the agency bring in leading psychedelics researchers.
We then dive into some of the specific challenges that psychedelic drug developers face and how Muniz, and the FDA, might think about them. Those include managing functional unblinding, demonstrating durability of effect, understanding the role of psychotherapy, and more. We also had Muniz weigh in on whether the agency has a higher bar for psychedelic programmes, if he thinks Janssen’s esketamine nasal spray (Spravato) would be approved today, and why Lykos Therapeutics’ MDMA-assisted therapy new drug application (NDA) was not approved earlier this year.
Aside from these more regulatory-minded topics, we also discuss Muniz’s views on mystical experiences (and whether their intensity makes attempts to blind psychedelics studies futile), decriminalisation and legalisation efforts, what the incoming Trump administration might mean for the field, and much more.
As you might have guessed, this interview is extensive. It’s our longest one yet at well over 7,000 words, so we have used subheadings, sometimes awkwardly, to help you navigate the discussion.
Without further ado, please enjoy Josh’s interview with Javier Muniz, MD…
***
In this Interview
- Muniz’s Background, Initial Interest in Psychedelics
- The Weight and Responsibility Associated with FDA Approvals
- Stoking Early Interest in Psychedelics at the FDA
- Early Discussions with Psychedelic Drug Developers, Esketamine’s Precedent, and Engaging with the Field
- FDA’s Internal Psychedelics Symposia, Guidance
- Would Spravato Be Approved Today? Debate Over Durability of Effect
- Does the FDA Have A Higher Bar for Psychedelics?
- Psychotherapy in Psychedelic Trials
- MAPS/Lykos Therapeutics’ ‘Bold’ Approach to Psychotherapy
- The Upshot for Sponsors: Minimise or Characterise Psychotherapy? (Or, Simplify the Story?)
- Non-Study Psychotherapy; The Role of Mystical Experiences
- Are Attempts to Blind Psychedelic Trials Futile?
- Why Did Lykos’ NDA Fail?
- Will a New U.S. Government Catalyse Psychedelic Drug Development?
- Views on Decriminalisation and Legalisation
- Comps for Psychedelic Drug Development
- Motivations for Continuing to Work in Psychedelics
***
Muniz’s Background, Initial Interest in Psychedelics
Josh Hardman, Psychedelic Alpha: Could you briefly sum up your interactions with psychedelic drug development at the agency?
Javier Muniz, MD: All my life has been a series of fortunate accidents. I ended up at the FDA about 11 years ago, starting in addiction and pain drugs. I loved it. I used to be a clinician in the military and taught psychopharmacology at the Uniformed Services University of the Health Sciences and Walter Reed. I had one student tell me: ‘You would be great at the FDA’ and I thought that it could be a great fit for me. When I got this job at the agency I thought it was amazing… people were paying me to learn stuff!
Then a friend of mine recruited me into the Division of Psychiatry because they were having significant turnover. Within a couple of months I started showing good work and having leadership potential, so they promoted me to Clinical Team Leader.
That’s when some of these psychedelic drugs started coming in more seriously, like MAPS (Lykos Therapeutics now, of course), then Compass Pathways and Usona Institute. I started getting really curious about it.
My initial draw to this field was more of an intellectual curiosity for trial design. I tend to immerse myself in reading whatever captures my interest, and when I began exploring the topic of psychedelics, it truly challenged my perspective. Until then, I had viewed psychedelics as relics of the past or simply grouped them with the notion that all drugs are inherently bad. I started exploring psychedelics and saw their potential, but I quickly encountered the issue of dramatic functional unblinding in trials. The key question is: how do we minimise these biases scientifically? So that’s what initially attracted me to this…
To read this interview, please sign in to your Pα+ account…
Join Pα+ Today
Independent data-driven reporting, analysis and commentary on the psychedelics space: from business and drug development through to policy reform and culture.
Already a member? Log In
✓ Regular Bulletins covering key topics and trends in the psychedelics space
✓ Regular articles and deep dives across psychedelic research, policy and business
✓ Interviews with insiders
✓ Monthly interactive database and commentary on psychedelic patents
✓ Quick-take analysis of major developments
✓ A Library of primers and explainers
✓ Access to our full back catalogue
As Donald Trump rounds out his nominations for key positions in his incoming administration, we’re keeping an eye on how his health-related picks might impact the future of the psychedelics field, notably drug development and policy reform efforts.
Of course, many of Trump’s picks will presumably need to be confirmed, but we will leave coverage of that process to other reporters.
Here, we review some key nominees from the past week or so, including any mentions of psychedelics, of a handful of health-related nominees…
FDA Pick – Marty Makary
Perhaps the biggest recent nominee-related news was that Martin (‘Marty’) Makary has been tapped as the next FDA commissioner and is thus set to replace Robert Califf. Many mainstream scientists and researchers have let out a sigh of relief, seeing Makary as a more moderate appointee than RFK Jr…
To continue reading, please log in or join Pα+…
Join Pα+ Today
Independent data-driven reporting, analysis and commentary on the psychedelics space: from business and drug development through to policy reform and culture.
Already a member? Log In
✓ Regular Bulletins covering key topics and trends in the psychedelics space
✓ Regular articles and deep dives across psychedelic research, policy and business
✓ Interviews with insiders
✓ Monthly interactive database and commentary on psychedelic patents
✓ Quick-take analysis of major developments
✓ A Library of primers and explainers
✓ Access to our full back catalogue
Earlier, we shared the high-level results of our Q3’24 Psychedelic Investor Survey, our third such survey. We also shared what investors are most excited about in the field.
Here, we take a closer look at investors’ qualitative responses to provide a better picture of their key areas of concern.
As a reminder, we allowed respondents to our Psychedelics Investor Survey to enter free-text responses to the question, “What are you most concerned about as an investor in psychedelics companies at present?” We then combed through their responses and tagged them according to a range of themes we identified.
In general, psychedelics investors are very concerned about regulatory and legislative barriers, with respondents of all stripes expressing this as the most salient area of uncertainty for the field. Retail investors continue to demonstrate sensitivity to company-level catalysts and drug development milestones, with trial timelines and outcomes a key concern among this cohort.
Larger investors, meanwhile, were more likely to express concerns over longer-term considerations like post-approval commercialisation factors or general commercial viability, as well as issues pertaining to safety and ethics. That latter category, safety and ethics, is a new one that emerged in this latest series of the survey.
Here, we take a deeper dive into these themes…
Contents
- Regulatory and Legislative Barriers
- Safety and Ethics
- Trial Outcomes and Development Timelines
- Funding (Sources and Uses)
- Commercialisation and Commercial Viability
- Stigma
- Other Topics
- ICYMI: What are Psychedelics Investors Excited About?
***
Regulatory and Legislative Barriers
While regulatory and legislative developments were formerly among the most cited reasons for excitement among psychedelics investors, this theme is now the primary cause for concern across investors of all stripes.
The responses generally have two flavours:
- a fundamental skepticism of the FDA and/or DEA’s willingness to approve and reschedule a psychedelic-based medicine; or,
- an acknowledgment that psychedelic drug development and commercialisation raise unique challenges for both developers and regulators.
i.e., the former comes from a place of skepticism of regulators’ appetite or political will to see psychedelics (re-)enter the medical field, while the latter focuses more on the agency and developers’ competency or remit.
The notion that the FDA is continuously ‘moving the goalposts’ regarding what it wishes to see in a psychedelic-based new drug application (NDA) was a popular one, expressed by everyone from VCs through to retail investors.
Through more in-depth interviews on this matter, it’s clear that some accept this is a natural part of the agency’s evolving understanding of the class of drugs as well as its developing standards for psychiatric drugs more broadly (e.g., greater scrutiny of things like functional unblinding and an expectation to see data on the durability of effect), while others believe it’s a deliberate attempt to skirt psychedelics’ approvability. Some investors reported a mix of these sentiments, suggesting that the two ‘flavours’ outlined above aren’t always discrete.
One retail investor cited the FDA as their biggest concern, “because its processes and programs are structured in such a way that psychedelic assisted treatment will never fit into their models.” Again, it’s hard to appreciate whether this is a concern about the agency’s remit (i.e., psychedelics don’t fit into its ‘models’) or indicative of fundamental skepticism about its willingness to approve a psychedelic (i.e., FDA is unwilling to adjust its ‘models’ to fairly appraise psychedelics).
This concern doesn’t always fall at the feet of the agency, however. One retail investor expressed concern about the sector’s ability to figure out “gaining FDA approval with the combo of drugs + psychotherapy”, adding that this is a “new frontier.” This investor wasn’t alone: a reasonable portion of respondents were not only concerned about the agency’s willingness or ability to appropriately assess a psychedelic-based therapy (especially a psychedelic-assisted therapy), but also companies’ (or, more nebulously, the ‘sector’s’) ability to play by the agency’s rules.
Similar sentiments were expressed by other investors who said, more plainly, that they are concerned about things like ‘FDA bureaucracy’, ‘government red tape’, and ‘over-regulation’. The DEA was also cited as a concern, though to a lesser extent and often indirectly, as was ‘the government’ more broadly.
Why has the mood shifted so much since our last survey? The most glaring reason is FDA’s rejection of Lykos Therapeutics’ NDA, as well as the hubbub in the run-up to the decision which included a very public airing of grievances and critiques at forums ICER’s review and the FDA Advisory Committee.
“The FDA-Lykos decision was really disheartening”, one family office investor responded, adding: “It really makes me wonder if anything can get approved.” Many other investors highlighted the Lykos decision as a cause for worry, which might have made concerns like those around the FDA’s willingness or ability to approve a psychedelic, and companies’ ability to play by the agency’s rules, particularly acute in this series.
While the majority of respondents cited very reasonable concerns around regulatory barriers to the approval and roll-out of psychedelics, a small minority shared more speculative sentiments. A few investors implied that ‘big pharma’ is plotting to prevent the market entry of psychedelics, while another said that there is ‘no money in healing.’
It is important to remember that our survey was carried out prior to the U.S. election, and that investor sentiment may have changed since that time.
According to a Psychedelic Alpha reader poll, 50% of respondents were pessimistic about the potential for psychedelics under a Trump administration, but 40% were optimistic and 10% were unsure. Another poll found that around half of our reader-respondents believe a Trump administration could catalyse psychedelic drug development. It is reasonable to think, then, that this most recent Psychedelic Investor Survey might over-index on concern in this realm…
To continue reading, please log in or join Pα+…
Join Pα+ Today
Independent data-driven reporting, analysis and commentary on the psychedelics space: from business and drug development through to policy reform and culture.
Already a member? Log In
✓ Regular Bulletins covering key topics and trends in the psychedelics space
✓ Regular articles and deep dives across psychedelic research, policy and business
✓ Interviews with insiders
✓ Monthly interactive database and commentary on psychedelic patents
✓ Quick-take analysis of major developments
✓ A Library of primers and explainers
✓ Access to our full back catalogue
Meet the group of scientists, activists and lawyers who came face-to-face with the DEA to challenge the agency’s proposed scheduling of two phenethylamine psychedelics, DOI and DOC.
Earlier, we shared the high-level results of our Q3’24 Psychedelic Investor Survey, our third such survey. Here, we take a closer look at investors’ qualitative responses to provide a better picture of their key areas of excitement. (We will explore key areas of concern in our third and final article detailing these survey results.)
As a reminder, we allowed respondents to our Psychedelic Investor Survey to enter free-text responses to the question, “What are you most excited about as an investor in psychedelics companies at present?” We then combed through their responses and tagged them according to a range of themes we identified.
In general, psychedelics investors of all stripes expressed most excitement around the discovery, development, and ultimate roll-out of new and effective therapeutics that can make a dent in the mental health crisis. This was particularly noticeable among retail investors but also family offices, which displayed a characteristically thematic or purpose-oriented motivation with regards to investing in the field.
VC and corporate investors showed a great deal of excitement around psychoplastogens (or, ‘non-hallucinogenic psychedelics’) and other ‘next-generation psychedelics’, as well as what they perceive to be a maturation or professionalisation of the field and its companies.
Retail investors often expressed excitement around specific drug development milestones and clinical trial readouts, as well as potential return on investment. This group of investors are particularly sensitive to near-term events.
Here, we discuss the various topics that psychedelics investors told us excited them…
Contents
- New and Effective Therapeutics
- Return on Investment
- Drug Development and Clinical Trial Results
- Consolidation, Maturation
- Psychoplastogens
- Commercialisation and Related Needs
- Care Delivery
- Other Topics
- Next: What are Psychedelics Investors Concerned About?
***
New and Effective Therapeutics
The promise of developing new and effective therapeutics remains the most commonly-cited cause for excitement among psychedelics investors we surveyed, but particularly resonates with retail investors.
Retail investors often reported excitement around supporting the emergence of ‘new and much-needed therapies for patients with a real need’. Accredited investors and family offices also mentioned this often, with family offices demonstrating a thematic interest in identifying ‘additional solutions to the massive mental health crisis’…
To continue reading, please log in or join Pα+…
Join Pα+ Today
Independent data-driven reporting, analysis and commentary on the psychedelics space: from business and drug development through to policy reform and culture.
Already a member? Log In
✓ Regular Bulletins covering key topics and trends in the psychedelics space
✓ Regular articles and deep dives across psychedelic research, policy and business
✓ Interviews with insiders
✓ Monthly interactive database and commentary on psychedelic patents
✓ Quick-take analysis of major developments
✓ A Library of primers and explainers
✓ Access to our full back catalogue
In this Issue
- Last-Minute Rule Changes, Opt-Outs, Ahead of Colorado’s Psychedelics Program Launch
- Cybin’s Impressive 12-Month Data Overshadowed by Small N and Controversial Marketing Move
- DEA Extends Telemedicine Rules for Controlled Substances Through 2025
- Vermont Psychedelic Therapy Advisory Working Group Kicks Can Down the Road
- Other Stories including: $PSY Sells Out, Raising $2 Million for Psychedelic Science; Johns Hopkins Launches Psilocybin Microdosing Study; New Industry Association Launches; Kentucky Ibogaine Initiative Figurehead Relocates Effort to REID Foundation; and Lawmakers in Georgia Consider Spending Up To $5 Million on Psychedelic Research for Veterans.
***
Last-Minute Rule Changes, Opt-Outs, Ahead of Colorado’s Psychedelics Program Launch
As Colorado’s state-regulated psychedelics system comes ever closer to its launch next year, last-minute changes to its rules are causing commotion.
With fewer than forty days to go until the state begins accepting license applications, and two years after voters endorsed Prop. 122 in the November 2022 elections, it’s crunch time.
Prop. 122 was seen as an improvement over Oregon’s restrictive first-in-class system, with notable changes including the prevention of local opt-outs and the leeway for psychedelic (natural medicine) sessions to take place outside of licensed facilities.
But, as the program’s launch draws closer, both of those USPs are coming under fire from rulemakers.
Narrowing the ‘Other Locations’ Rules
Last-minute additions to section 6.18 of the final draft rules specify that a facilitator that is “employed by, contracted with, or holds a healing center license may facilitate an administration session in a location other than a healing center”, but only “if they are facilitating for a participant requiring palliative care.” (The previous text of 6.18 A. read, simply, “A facilitator may facilitate an administration session in a location other than a healing center in accordance with these rules.”)
The employment or licensure clause, and the palliative care restriction, were the two elements added very late in the day: the changes were made last Friday, and the final hearing took place on Tuesday.
Prior to this change, at-home administration was largely unrestricted, aside from basic safety stipulations. But according to these updated draft rules, such administration would be narrowly limited to individuals undergoing palliative care. The new wording also foils the whole ‘independent facilitator’ set-up that was set to be one of the Colorado program’s more radical features, which advocates hoped would increase inclusion and reduce barriers to access…
Sign-in or join Pα+ to continue reading this Issue of the Psychedelic Bulletin…
Join Pα+ Today
Independent data-driven reporting, analysis and commentary on the psychedelics space: from business and drug development through to policy reform and culture.
Already a member? Log In
✓ Regular Bulletins covering key topics and trends in the psychedelics space
✓ Regular articles and deep dives across psychedelic research, policy and business
✓ Interviews with insiders
✓ Monthly interactive database and commentary on psychedelic patents
✓ Quick-take analysis of major developments
✓ A Library of primers and explainers
✓ Access to our full back catalogue
Topline results from the latest in our series of psychedelics investor surveys. For detailed analysis, subscribe to Pα+ today.
In a quiet courtroom housed on the second floor of the Drug Enforcement Agency’s (DEA) Museum and Visitors Center in Arlington, Virginia, a legal battle is unfolding over the status of two key psychedelic research chemicals: DOI and DOC. Here, Jack Gorsline and Josh Hardman provide the latest from the first week of hearings over the DEA’s plan to place these two psychedelics in Schedule I of the Controlled Substances Act.
In this Issue
- Massachusetts’ Psychedelics Ballot Question Post-Mortem Begins
- Cybin Commences Phase 3 Program of Deuterated Psilocin, CYB003, in Major Depressive Disorder
- Opt-Out Trend Continues: Oregon’s Local Jurisdictions Push Back on Psilocybin Access
- CaaMTech Claims It’s Solved Functional Unblinding, But Won’t Tell Us How
- Compass Says Phase 3 Readout Punt Is About Durability, Not Unblinding
- Lykos-Funded Study Finds MDMA-AT Cost Effective with Drug Priced at $12k per Session
- Other Stories including: RFK Jr. Scores HHS Position; UC Davis Researchers Claim Separation of DOI’s Hallucinogenic and Anxiolytic Effects; Relmada Launches Ph 1 Study of Low-Dose Psilocybin; Numinus Sells Clinics; and more.
***
Massachusetts’ Psychedelics Ballot Question Post-Mortem Begins
Last-minute donations from Dr. Bronner’s ($100k), Elliot Cohen ($100k), Eliza Dushku ($100k), MAPS ($50k), and others could not push Question 4 over the line, with the Massachusetts ballot initiative suffering defeat by a convincing margin. (See Question 4 Fails: Massachusetts Says No to State-Regulated Psychedelics Access and Decriminalisation.)
The Yes campaign, organised under Massachusetts for Mental Health Options (MMHO), hosted a results party on election night, but the event was decidedly private, with our assigned reporter on the ground hoofed out of the do. (He wasn’t the only journalist to suffer that fate, with Boston’s local NPR outlet’s reporter shown the door, too!)
Now, the post-mortem has begun.
Sam Chapman, who is currently the Policy and Development Director at the Microdosing Collective but previously led on the development and roll-out of state-level psychedelic programs at Healing Advocacy Fund, believes the ballot question was too complex.
“With Question 4, the title had to cover decriminalization, home cultivation, personal possession, and a regulated program—all complex issues bundled together”, Chapman told us. “This broad scope may have contributed to voter hesitation”, he added, suggesting that future attempts should have “ballot titles that clearly convey the intent without overwhelming voters.”
Indeed, we had heard this from those on the ground in Massachusetts throughout the campaign, and mentioned it several times in our own commentary.
MAPS’ Rick Doblin believes that the failure in Massachusetts can be partially attributed to a broader public narrative shift around psychedelics, one that’s gone “from hope to caution”. Echoing sentiments expressed during Lykos Therapeutics’ new drug application review process, Doblin told us that he believes the company “lost control over the public narrative”, with the FDA rejection and associated negative press “further diminishing public confidence and support.” He was also keen to point out Compass Pathways’ recent layoffs and Phase 3 readout delay as another example of a challenging time for the field.
The results in Mass. “suggest it will be four years or more” before another initiative goes before Bay Staters, Doblin said, urging proponents to focus on more public education and to be hopeful for promising data out of Oregon and Colorado’s programs.
Others have accused New Approach PAC of failing to engage sufficiently with grassroots groups or to reach local people, with many voters unaware of the question’s existence until they were confronted with it on the ballot.
For its part, New Approach looks keen to shift blame onto those very grassroots groups. “We understand there were concerns about the home grow provisions, and those concerns likely led to tonight’s result,” it said in immediate response to the results. Those home grow provisions, of course, were of tantamount importance to many grassroots psychedelics groups in the state.
Will this experience lead New Approach to reevaluate its strategy, or will it encourage it to push ahead with a more top-down approach to state-by-state psychedelic drug policy reform? More to come.
***
Cybin Commences Phase 3 Program of Deuterated Psilocin, CYB003, in Major Depressive Disorder
Cybin has become the latest drug developer to take a psychedelic into Phase 3, with its Wednesday announcement that its PARADIGM program has commenced.
The program consists of two Phase 3 (APPROACH and EMBRACE) as well as an open-label extension study (EXTEND) that takes over after week 12 in both of the core studies. Each of the trials will evaluate the company’s deuterated psilocin candidate, CYB003, in patients with moderate to severe major depressive disorder (MDD; MADRS scores ≥24) who are on a stable dose of antidepressants but are not responding sufficiently.
CEO Doug Drysdale characterised the FDA review process as “highly collaborative and thorough”, and described several design features that he hopes will assuage the agency’s concerns and prove out the potential scalability of CYB003 in MDD. Those include…
Sign-in or join Pα+ to continue reading this Issue of the Psychedelic Bulletin…
Join Pα+ Today
Independent data-driven reporting, analysis and commentary on the psychedelics space: from business and drug development through to policy reform and culture.
Already a member? Log In
✓ Regular Bulletins covering key topics and trends in the psychedelics space
✓ Regular articles and deep dives across psychedelic research, policy and business
✓ Interviews with insiders
✓ Monthly interactive database and commentary on psychedelic patents
✓ Quick-take analysis of major developments
✓ A Library of primers and explainers
✓ Access to our full back catalogue
As the psychedelics field licks its wounds after a string of defeats in recent months, many believe they have found a silver lining in the form of an incoming Trump administration. But while there are several prominent psychedelics advocates in the President-elect’s orbit, it’s not yet clear whether, or how, the new administration would catalyse psychedelic drug development or policy reform. Here, we take a deep dive…
Massachusetts voters have rejected Question 4, the only standalone psychedelic policy reform initiative to go before the electorate during this year’s polls. That means the Bay State will not become the third to establish a state-regulated psychedelics system, or decriminalise the use, possession and home growth of certain psychedelics.
INCUBATION & GROWTH
Psychedelic Insiders and the Curious
Join our newsletter to stay abreast of the rapidly emergent psychedelics industry: from clinical trials and patents through to healing centres and decriminalisation.
ADVISORY & CONSULTANCY
Investors and Corporates
We help investors and corporates of all stripes understand and support the psychedelics space: from syndicates and VCs through to institutions.
INCUBATION & GROWTH
Entrepreneurs and Startups
Providing consultancy, advisory, and incubation services for investors and entrepreneurs in the psychedelic sector.
The Psychedelic Renaissance.
After a half-century of prohibition, psychedelics are once again entering the mainstream.
Today’s so-called “psychedelic renaissance” is represented by a seemingly exponential increase in research and development activity, billions of dollars of funding, psychedelic policy reform initiatives and successes across the world, and changing public perceptions.
Clinical Trials
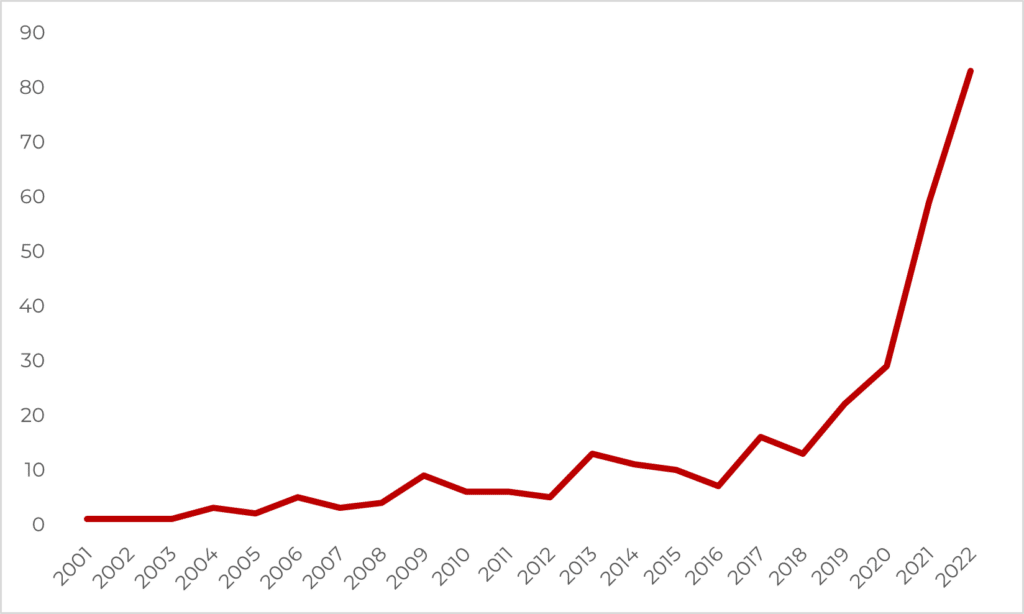
Phase 3 trials of MDMA-assisted therapy have been completed, with Phase 3 study of psilocybin underway.
Investment
Invested in public and private psychedelic companies since 2020.
Policy Reform
Psychedelic policy reforms are gaining pace at the federal, state and local level in the U.S. and internationally.
Perceptions
of psychiatrists agreed that psychedelics show promise in treating psychiatric disorders.
On “Alpha”
‘Alpha’, in the investing world, refers to an excess return on investment relative to a benchmark. But we broaden the definition of alpha on both ends to include more than just economic returns on more than just economic investments.
Instead, we conceptualise alpha as the excess return on myriad investments that people may make in the present and future of psychedelics: beyond economic capital, people might invest time, energy, and effort, for example. And that excess return might not be (purely) financial, it might also be realised through a more impactful treatment paradigm for mental health, for example, where the ‘benchmark’ is the inadequate status quo.
All this to say, we’re not only for investors. In fact, most of our readers aren’t: many of them are practitioners, regulators, entrepreneurs, and so on. ∎

